Ainprose™
Our clinic offers therapy using Ainprose™ with strict quality control across all stages from manufacturing to clinical application.
[Therapy Offered] Adjunctive therapy with Ainprose™
This therapy is not covered by health insurance. *One of the risks associated with this therapy is that the administration of the Ainprose™ may cause side effects such as temporary allergies.
In Japan and other countries, there is no definitive information on the potential for serious risks associated with Ainprose™.
"Ainprose™ infusion therapy" is required to be performed as a medical procedure under the responsibility of medical institutions and physicians, and "Ainprose™" itself is not a drug approved
under the Pharmaceutical Affairs Law.
[Contact] Sinclair Ginza Clinic 4F & 5F FPG Links Ginza, 2-8-19 Ginza, Chuo-ku, Tokyo, 104-0061 Phone: +81-3-3538-6082
〈Those who are unable to receive treatment〉This therapy is unavailable to pregnant women or individuals undergoing cancer treatment.
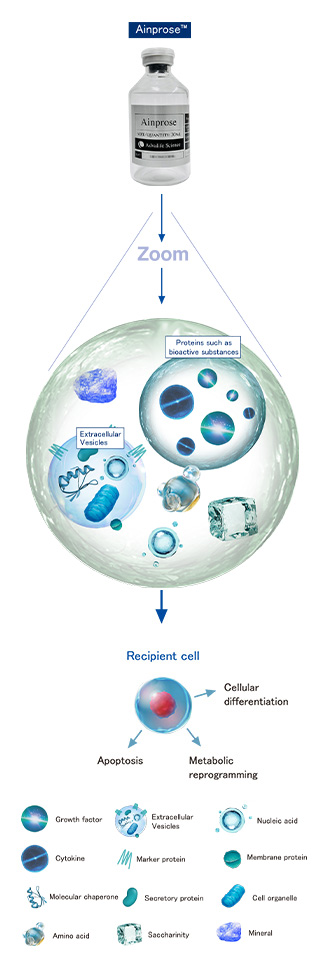
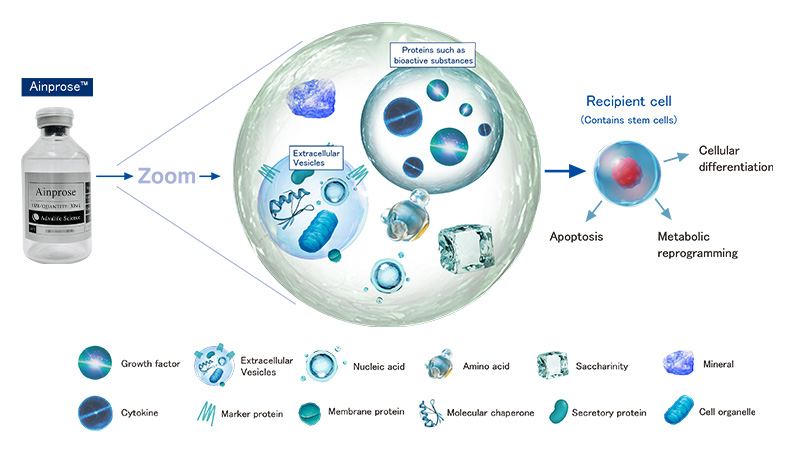
Ainprose™
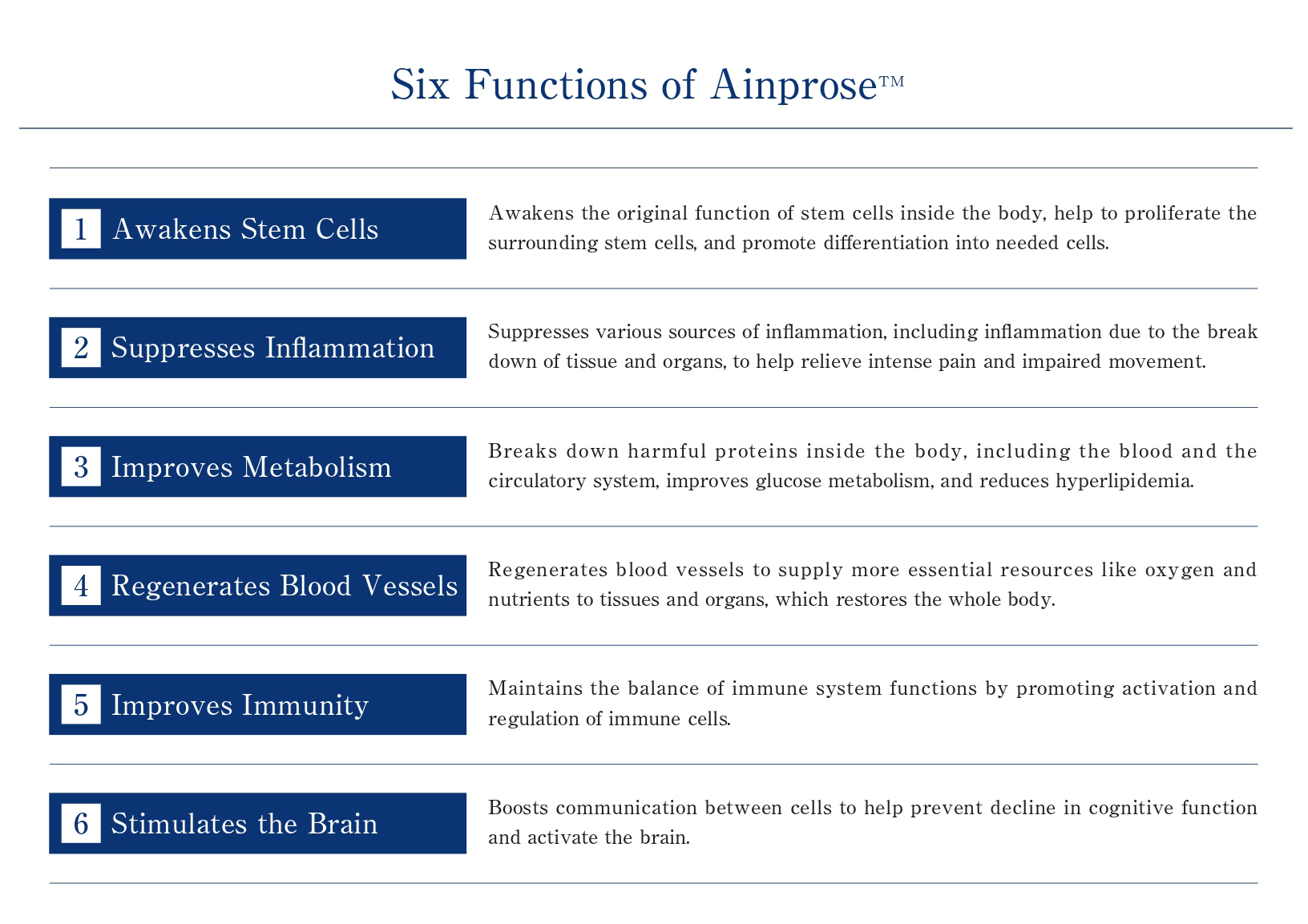
Ainprose™ contains bioactive substances,
which act on cells to promote tissue regeneration,
and there are growing expectations for its effectiveness.
*Ainprose™ does not contain any cells.
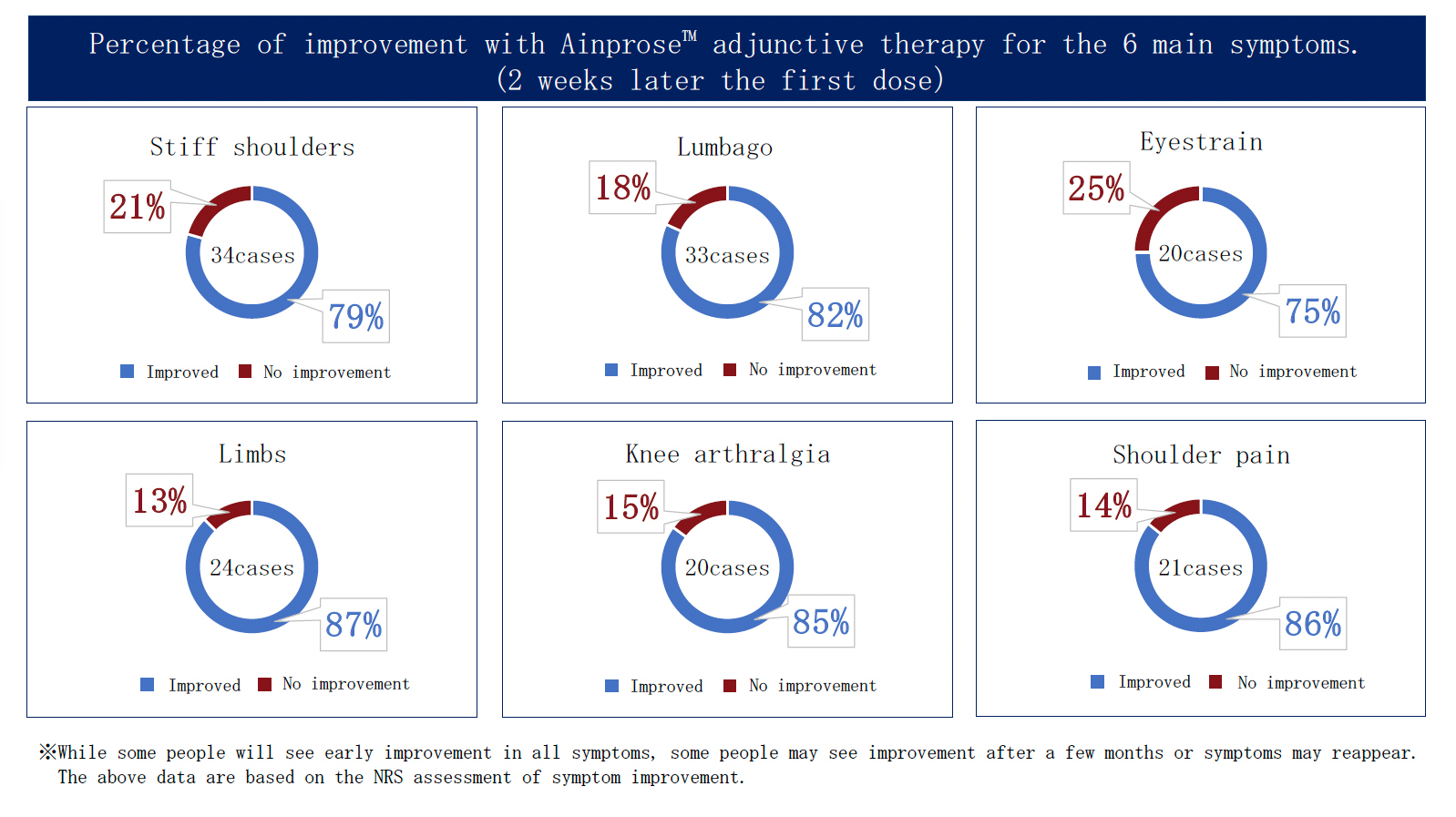
Ainprose™ therapy (30 mL infusion) Total results
Number of doses: 3,000 total
(as of Jan 2025)
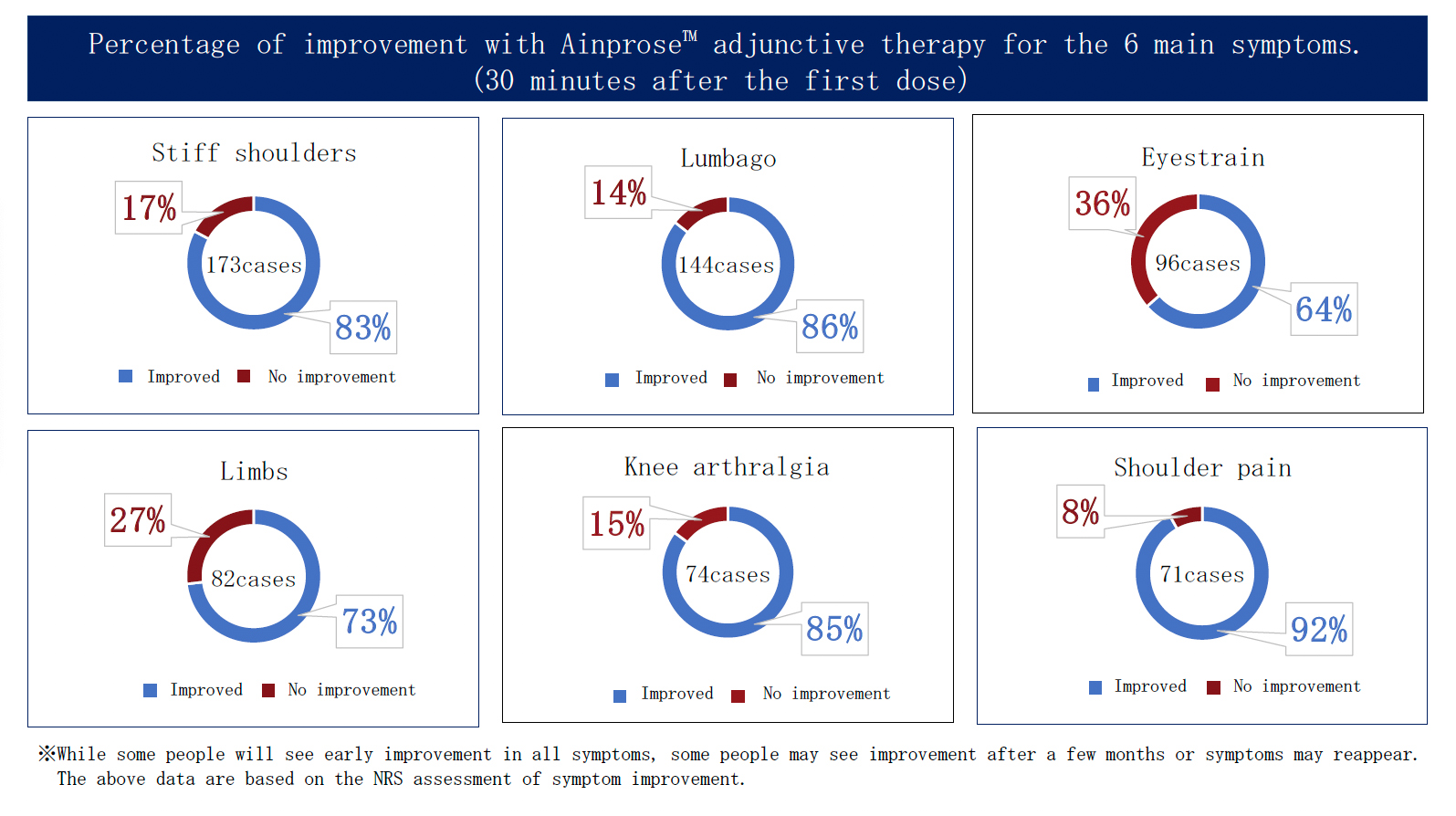
Relief for Many Symptoms
- Bone, Muscle, Joint Symptoms
- Osteoarthritis
- Gout
- Hernia
- General stiffness (stiff back)
- Stiff shoulders
- Frozen shoulders
- Strained back
- Bone fractures
- Torn muscles
- Heberden's nodes
- Wrist tendonitis
- Neurological Symptoms
- Numbness
- Motor paralysis
- Sensory abnormalities
- Diabetic neuropathy
- Systemic Symptoms
- Headaches
- Brain fog
- Fatigue
- Dizziness/
lightheadedness - Insomnia/night sweats
- Chronic pain
- Rheumatic diseases
- Promote wound healing
- Improve post-op after effects
- Poor immunity
- Swelling
- Hangovers
- Vascular Symptoms
- Arrhythmia
- Peripheral circulation disorders
- Arteriosclerosis
- Vision Symptoms
- Eye strain
- Impaired vision
- Presbyopia
- Floaters
- Diabetic retinopathy
- Skin Symptoms
- Rough skin
- Vitiligo
- Rashes
- Burn scars
- Liver Pancreas Symptoms
- Dyslipidemia
- High blood sugar
- Respiratory Symptoms
- Pneumonia
- Asthma
- Urological Symptoms
- Frequent urination
- Urinary incontinence
- Erectile dysfunction (ED)
- Gynecological Symptoms
- Infertility
- Menopausal disorder
Video of Improvement Cases at our clinic
※Effects vary from person to person
[Therapy Offered] Adjunctive therapy with Ainprose™
This therapy is not covered by health insurance. *One of the risks associated with this therapy is that the administration of the Ainprose™ may cause side effects such as temporary allergies.
In Japan and other countries, there is no definitive information on the potential for serious risks associated with Ainprose™.
"Ainprose™ infusion therapy" is required to be performed as a medical procedure under the responsibility of medical institutions and physicians, and "Ainprose™" itself is not a drug approved
under the Pharmaceutical Affairs Law.
[Contact] Sinclair Ginza Clinic 4F & 5F FPG Links Ginza, 2-8-19 Ginza, Chuo-ku, Tokyo, 104-0061 Phone: +81-3-3538-6082
〈Those who are unable to receive treatment〉This therapy is unavailable to pregnant women or individuals undergoing cancer treatment.

Proprietary product manufacturing facility
"Sinclair Ginza Clinic" manufactures Ainprose™ in its own product manufacturing facility, which has been performing production involving approximately 100,000 cultures without incident for 20 years under thorough safety and quality control.

Adjunctive therapy with Ainprose™
- Vascular Regeneration
and Angiogenesis - Collateral angiogenesis for interrupted blood circulation due to atherosclerosis
- Nerve Cell Repair
and Regeneration - Repair of peripheral nerves
- Anti-Inflammation Effects
- Promotes healing of damaged areas (injury and inflammation) and reduces pain
- Anti-Oxidant Effects
- Recovery from fatigue, prevention of lifestyle diseases
- Immune System Regulation
- Allergies and sensitivities
- Cosmetic Effects
- Improve wrinkles and sagging with tissue repair actionby repairing tissue
Growth factors contained in Ainprose™
- EGF
(Epidermal Growth Factor) - These growth factors are composed of amino acids that promote the production of new cells, which can act on various cells to encourage growth of cells, regulate proliferation, and make repairs.
- VEGF
(Vascular Endothelial Growth Factor) - These growth factors play a key role in angiogenesis and induce cell division and differentiation, which causes the formation of new blood vessels that branch off from existing ones.
- TGF-β
(Transforming Growth Factor) - These growth factors regulate cell proliferation, growth, differentiation, and motility, playing key roles in the reconstruction, wound healing, inflammation, and immunity of cellular tissues.
- HGF
(Hepatocyte Growth Factor)) - These growth factors promote the proliferation of hepatocytes involved in the synthesis and storage of proteins, the conversion of carbohydrates, and the detoxification, denaturation, and elimination of cholesterol and xenobiotics.
- KGF
(Keratinocyte Growth Factor)) - These growth factors are mitogenic (induce cell division) with angiogenesis and wound healing effects, which assist in the production of keratin, an essential ingredient the formation of hair and skin.
- IGF
(Insulin Growth Factor)) - These growth factors are produced by the growth hormone which is secreted in large quantities during puberty. They function in a variety of ways, including the promotion of cell proliferation/differentiation, and protein assimilation.
- PDGF
(Platelet-Derived Growth Factor) - These growth factors are produced by various cells such as epithelial and endothelial cells. They are involved in regulating the migration and proliferation of mesenchymal cells, which helps promote the growth and regeneration of damaged tissues.
- FGF
(Fibroblast Growth Factor) - These growth factors are involved in angiogenesis and wound healing.
They play key roles in the proliferation and differentiation of a wide range of cells and tissues.
- IL-7
(Interleukin-7)) - These regulating factors are bioactive substances that promote cell proliferation, and are keys to the survival, proliferation and maturation of cells.
- GM-CSF
(Granulocyte-Monocyte Colony-Stimulating Factor)) - These growth factors promote cell survival and activation. They are hematopoietic growth factors that promote the differentiation into pluripotent hematopoietic stem cells and function as immunoregulatory factors.
- EPO
(Erythropoietin)) - These growth factors are mainly produced in the kidneys, and are one of the hematopoietic factors that promote the production of red blood cells.
- TSG-6
(Anti-Inflammatory Factor) - These growth factors are gaining attention for suppressing inflammation, and as a treatment for arteriosclerosis.
- TPO
(Thrombopoietin)) - These hematopoietic factors are involved in the proliferation and differentiation of proplatelets, and are considered to be key factors in the production of hematopoietic cells.
- TIMP
(Tissue Inhibitor of Metalloproteinase) - MMP Inhibits the degradation of tissue collagen and elastin by MMPs, and helps prevent fibrosis.
※MMPs (matrix metalloproteinases) are enzymes that degrade intercellular proteins such as collagen, proteoglycans, and elastin. Abnormal MMPs are said to be correlated with the development of problems including cancer, inflammation, multiple sclerosis, and gum disease.

Safety of Ainprose™
- Manufactured in a strictly controlled facility
- Sterilization in clean rooms and prevention of contamination
Doctor introduction





MEDICAL MENU
Holders of a Japanese health insurance card or a resident card in Japan
Adjunctive Therapy Featuring Ainprose™ (Intravenous Infusion / Intravenous Injection)
- Intravenous infusion
-
Ainprose™ 30mL: 1 treatment
¥450,000 w/o tax
(495,000 yen (including tax))
- Intravenous infusion
-
Ainprose™ 30mL: 3 treatments
¥1,215,000 w/o tax
(1,336,500 yen (including tax))
※Therapy Period: 1 year from the day therapy starts
- Intravenous infusion
-
Ainprose™ 30mL: 6 treatments
¥2,250,000 w/o tax
(2,475,000 yen (including tax))
※Therapy Period: 2 years from the day therapy starts
- Intravenous infusion
-
Ainprose™ 10mL: 1 treatment
¥205,000 w/o tax
(225,500 yen (including tax))
- Intra-articular Injection
-
Ainprose™ 5mL: 1 treatment
¥115,000 w/o tax
(126,500 yen (including tax))
Adjunctive Therapy Featuring Ainprose™ (Targeted Administration)
- Intra-articular Injection
-
Ainprose™ Treatment: 1 location
¥90,000 w/o tax
(99,000 yen (including tax))
Those who do not have a Japanese health insurance card or a resident card in Japan
Adjunctive Therapy Featuring Ainprose™ (Intravenous Infusion / Intravenous Injection)
- Intravenous infusion
-
Ainprose™ 30mL: 1 treatment
¥671,000(including tax)
- Intravenous infusion
-
Ainprose™ 30mL: 3 treatments
¥2,013,000(including tax)
Re:A Set of 4 cosmetics
※Therapy Period: 1 year from the day therapy starts
- Intravenous infusion
-
Ainprose™ 30mL: 6 treatments (+1)
¥4,026,000(including tax)
Re:A Set of 4 cosmetics
Ainprose™ 30mL: 1 additional treatment valued at ¥671,000 added at no extra cost.
※Therapy Period: 2 years from the day therapy starts
- Intravenous infusion
-
Ainprose™ 10mL: 1 treatment
¥308,000(including tax)
- Intra-articular Injection
-
Ainprose™ 5mL: 1 treatment
¥170,500(including tax)
Adjunctive Therapy Featuring Ainprose™ (Targeted Administration)
- Intra-articular Injection
-
Ainprose™ Treatment: 1 location
¥134,200(including tax)
[Therapy Offered] Adjunctive therapy with Ainprose™
This therapy is not covered by health insurance. *One of the risks associated with this therapy is that the administration of the Ainprose™ may cause side effects such as temporary allergies.
In Japan and other countries, there is no definitive information on the potential for serious risks associated with Ainprose™.
"Ainprose™ infusion therapy" is required to be performed as a medical procedure under the responsibility of medical institutions and physicians, and "Ainprose™" itself is not a drug approved
under the Pharmaceutical Affairs Law.
[Contact] Sinclair Ginza Clinic 4F & 5F FPG Links Ginza, 2-8-19 Ginza, Chuo-ku, Tokyo, 104-0061 Phone: +81-3-3538-6082
〈Those who are unable to receive treatment〉This therapy is unavailable to pregnant women or individuals undergoing cancer treatment.
The Sinclair Ginza Clinic is a medical institution that can provide regenerative medicine in accordance with the regenerative medicine provision plan, having submitted and received approval for a Type 2 regenerative medicine provision plan from the Ministry of Health, Labour and Welfare.
Type 2 Regenerative Medicine Provision Plan Already submitted
Plan number: PB3230226
Map/Hours

Sinclair Ginza Clinic
4F & 5F FPG Links Ginza, 2-8-19 Ginza, Chuo-ku, Tokyo, 104-0061
Ginza-itchome Station [Exit 9]: 1 minute walk
Ginza Station [Exit A13]: 4 minutes walk
Higashi-ginza Station [Exit A8]: 6 minutes walk
JR Yurakucho Station [Kyobashiguchi Exit]: 7 minutes walk

| Clinic Hours | Mon | Tue | Wed | Thu | Fri | Sat | Sun |
|---|---|---|---|---|---|---|---|
| 10:00 AM to 1:00 PM | 〇 | 〇 | Closed | 〇 | 〇 | 〇 | Closed |
| 2:00 PM to 7:00 PM | 〇 | 〇 | Closed | 〇 | 〇 | 〇 | Closed |
Last Check-in: 6:00 PM